Electrodialysis for Salt Purification
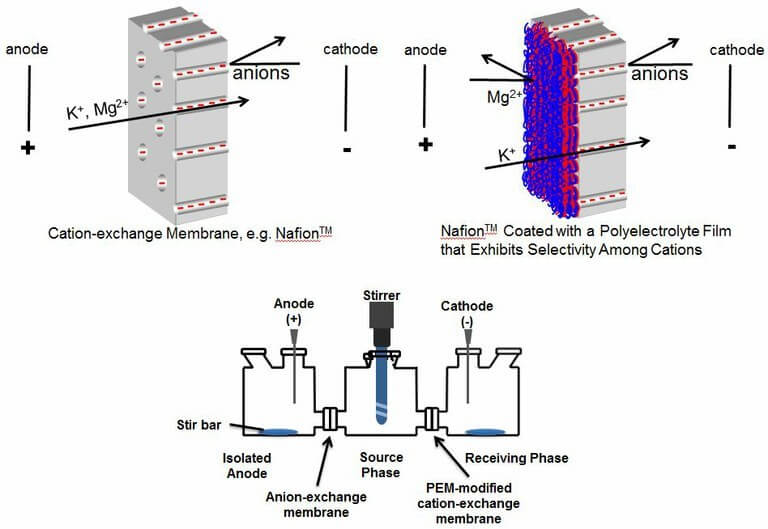
In electrodialysis, an electrical potential drives ions across membranes to effect separations. However, typical ion-exchange membranes show minimal selectivity between monovalent and divalent ions, which is important for applications such as salt purification. In contrast, we found that polyelectrolyte multilayers adsorbed on ion-exchange give rise to an electrodialysis K+/Mg2+ selectivity >1000. Recently we showed that these membranes can separate K+ and Mg2+ or Li+ and Mg2+ to give 99.9% pure K+ or Li+ with recoveries ranging from 60-80% J. Membr. Sci. 647, 120294 (2022). Development of a careful current program is important for maintaining high efficiencies, and separations can occur with low energy costs. Coated membranes also show high Li+/Co2+ selectivities, which might prove useful in recycling Li batteries.
The Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy (DE-FG02-98ER14907) supports our efforts in electrically-driven separations.
