Counter-Flow Electromigration Separations of Monovalent Ions
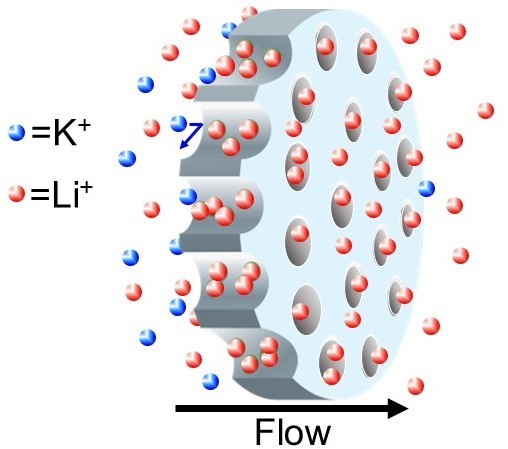
Ion separations are vital for purifying the salts needed to produce batteries, magnets, and other devices. Graduate student Chao Tang's research demonstrates that simple flow of ionic solutions through charged nanoporous membranes can separate dilute ions based on their different electrophoretic mobilities. Remarkably, flow of solutions containing LiCl and KCl through a polycarbonate membrane with 30-nm pores gives a Li+/K+ selectivity of 50 Chem. Commun., 56, 10954-10957 (2020).
The separation mechanism includes development of a streaming potential that causes cation electromigration that opposes the convective flow through the membrane. Because the membrane pores are negatively charged and contain an excess of cations, flow through the membrane induces a transmembrane potential drop that maintains zero current. This potential retards the transport of K+ more than that of Li+ because K+ has a larger electrophoretic mobility than Li+. At sufficiently fast flow the separation becomes highly selective even with a relatively high Li+ passage. Unfortunately, the separation only occurs at salt concentrations low enough to give high streaming potentials. Current work aims to use electrodes to perform separations at higher salt concentrations. We recently showed that when applying an electrical potential across the membrane Li+/K+ selectivities around 150 are possible even at high ionic strength J. Membrane Sci. 638, 119684 (2021). However, the separation now requires electrodes and energy input.
We are grateful for funding from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of energy through Grant DE-SC0017618.
